Bile acids represent a unique subset of biochemical compounds that fall under the broader category of steroids. Despite their association with negative health issues like cardiovascular disease or misuse of hormones, bile acids are crucial for cellular health. Steroids, led by cholesterol, serve vital roles in maintaining cell structure and function, acting as regulators, precursors, and mediators of numerous physiological processes.
As suppliers of fine chemicals, we aim to provide a concise yet comprehensive introduction to bile acids, given the complexity of the topic. For deeper insights, readers should consult specialized literature.
Structure of Bile Acids
Bile acids are classified as steroids, meaning their molecular structure features a 4-ring cyclopentane-perhydrophenanthrene system, often referred to as "gonane" or "sterane." The backbone of this structure is similar across all bile acids but can vary slightly depending on functional groups attached to it.
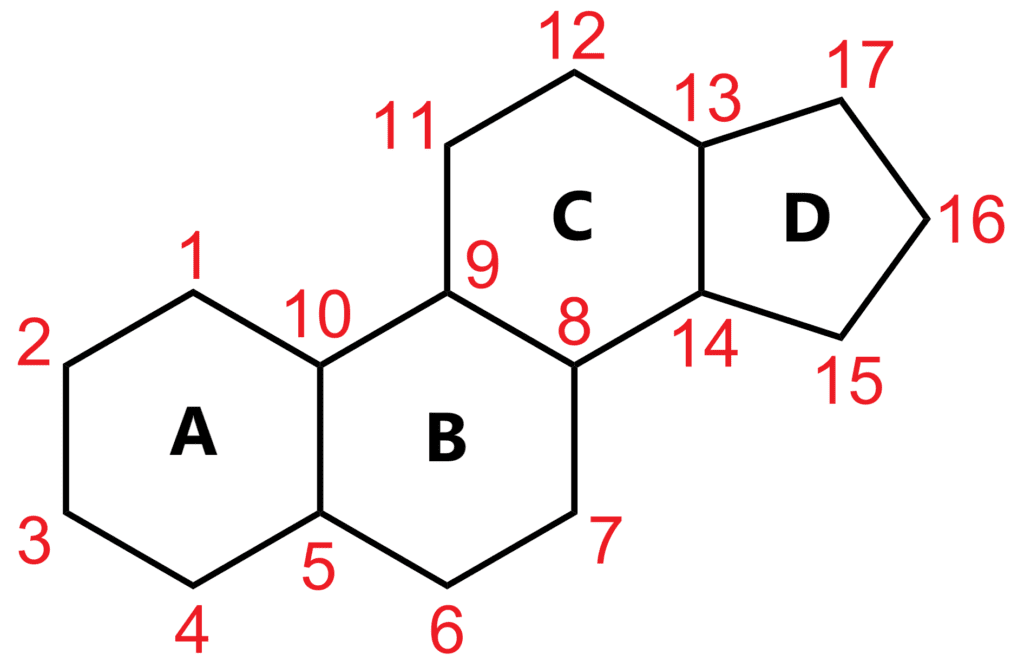
This illustration shows the basic skeleton of a sterane molecule, the simplest form of a steroid. The carbon atoms are numbered according to IUPAC guidelines.
The A and B rings typically fuse in a cis configuration, though the A ring can adopt either a chair (more stable) or ship (less stable) conformation. Meanwhile, the B/C and C/D rings are fused in a trans configuration. These structural variations influence how bile acids interact with biological systems.
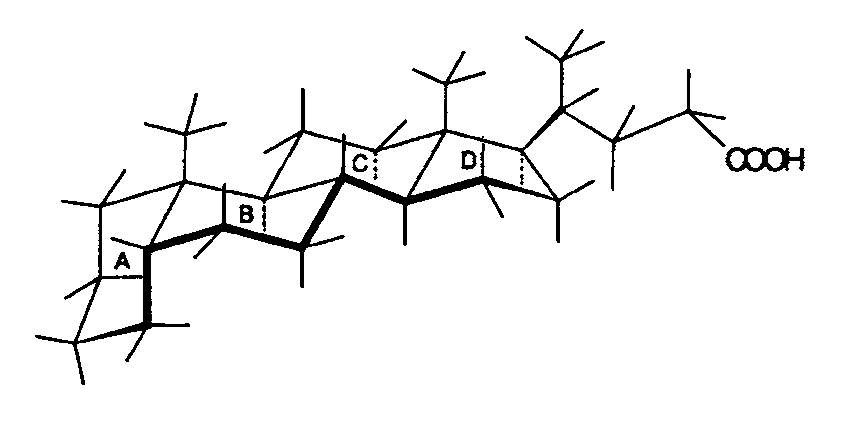
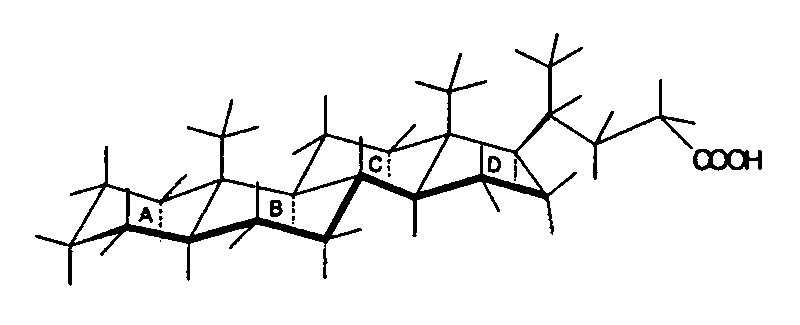
These images depict the structures of 5a-cholanic acid (left) and 5b-cholanic acid (right), highlighting the impact of A/B cis and A/B trans configurations on the overall shape of the molecule.
Functionally, bile acids often contain hydroxyl groups located at specific carbons, such as C3, C6, C7, C12, and C23. Methyl groups are also commonly found at C10 and C13. The stereochemistry of these compounds is particularly significant, as the orientation of methyl groups at C19 determines whether hydrogens at C5 and C8 exhibit cis or trans configurations.
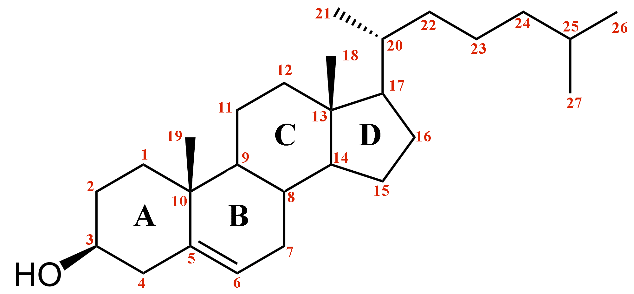
The cholesterol molecule, showcasing the carbon skeleton numbering scheme.
At the C17 position, bile acids typically feature an aliphatic chain with varying functionalities. Factors such as the length and functionality of this chain, the presence of methyl and hydroxyl groups, and the overall stereochemistry help distinguish one steroid from another.
Biosynthesis and Classification
Cholic acid serves as the foundational bile acid, from which derivatives are created through hydroxylation at different carbon positions. Although cholanic acid exists naturally, mammals primarily rely on cholesterol as the precursor for bile acid production. Cholesterol, despite its hydrophobic nature, becomes a bile acid when the terminal end of its aliphatic chain is oxidized. Liver cells perform this transformation, yielding primary bile acids—cholic acid and chenodeoxycholic acid:
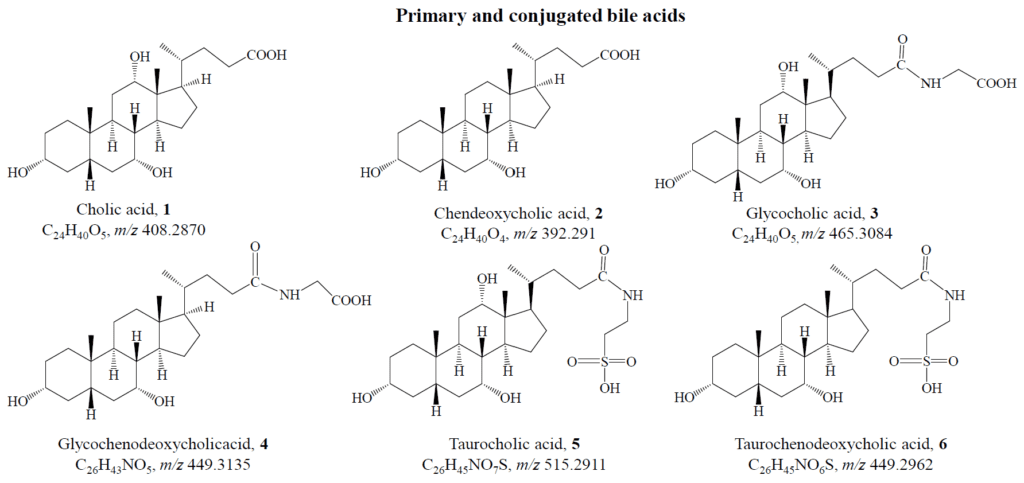
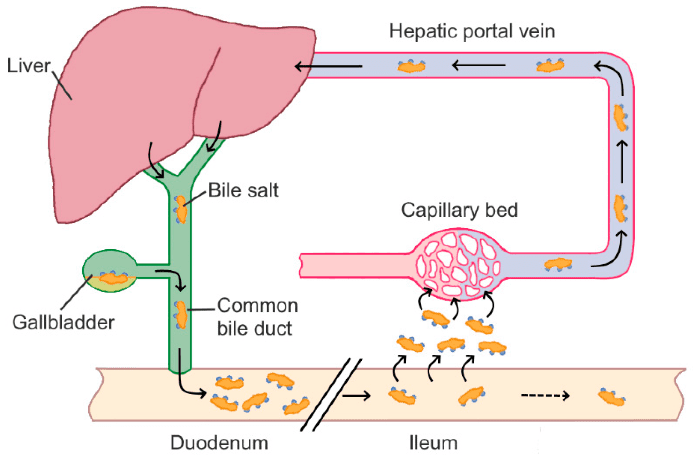
These acids travel to the digestive tract via the gallbladder, reaching the duodenum, where the pH ranges between 5 and 6.5. However, the pKa of these acids lies between 3 and 5, making them predominantly protonated and poorly water-soluble. To address this issue, the liver conjugates these acids with glycine or taurine, forming ionic pairs that enhance solubility in aqueous environments.
The intestinal microbiota then steps in, breaking down these ionic pairs and converting the acids into secondary bile acids, primarily through dehydroxylation at the C7 position. Further modifications, such as epimerization or conjugation with N-acetylglucosamine, lead to tertiary bile acids.
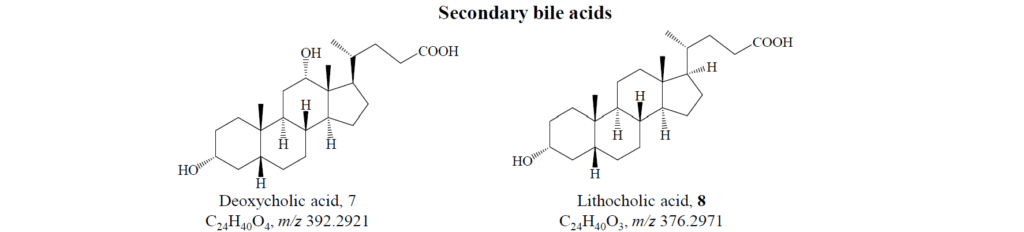
Ultimately, these acids can be reabsorbed from the gut and recycled back to the liver, a process known as enterohepatic circulation:
Functions of Bile Acids
Bile acids exhibit surface-active properties in neutral or basic environments, with the aliphatic end containing the carboxyl group acting as the hydrophilic region, while the rest of the molecule remains lipophilic. Their primary role is to emulsify fats and non-polar substances into micelles, enabling absorption by the small intestine and ensuring solubility in the bloodstream. This facilitates the action of lipases, enzymes responsible for breaking down lipids into free fatty acids and glycerol.
Beyond their emulsifying capabilities, bile acids also serve as chemical mediators by binding to various cell receptors to initiate critical biochemical pathways (cell signaling). They play a role in regulating inflammation, calcium mobilization, protein kinase activation, cyclic AMP synthesis, and pro-inflammatory cytokine secretion. Additionally, they participate in mitochondrial oxidative processes and insulin receptor signaling. Furthermore, bile acids help control cholesterol levels and contribute to the elimination of catabolites like bilirubin.
To sum up, bile acids fulfill numerous functions, and while cholesterol might raise concerns, its presence is vital for proper bodily function when maintained within appropriate limits. The challenge lies in managing cholesterol intake effectively.
Â
Â
 References:
- Kuhajda, K. et al, “Structure and origin of bile acids: an overview,†European Journal of Drug Metabolism and Pharmacokinetics 2006, Vol. 31, No.3, pp. I3S-143.
- Chiang, J.Y.L., “Bile acids: regulation of synthesis,†Journal of Lipid Research, Volume 50, 2009, 1955-66.
- Singh, J. et al., “A Review on Bile Acids: Effects of the Gut Microbiome, Interactions with Dietary Fiber, and Alterations in the Bioaccessibility of Bioactive Compounds,†J. Agric. Food Chem., Just Accepted Manuscript • DOI: 10.1021/acs.jafc.8b07306.
Medical Consumables,Consumable Medical,Healthcare Consumables,Medical Device Consumables
Shandong Zhushi Pharmaceutical Group Co.,LTD , http://www.sdzs-medical.com