Li Jianzhong, Jin Qiwei, Yap Swee Lee Waters Corporation, Shanghai, China
summary
This article uses Waters® Oasis® HLB solid phase extraction technology and ACQUITY UPLC® Ultra Performance Liquid Chromatography/TQD Tandem Mass Spectrometry System to analyze glucocorticoid stimulants in animal foods.
Foreword
The chemical structure of glucocorticoids is a steroid compound that regulates glucose metabolism, promotes protein conversion to sugar, enhances blood glucose concentration, resists inflammation, anti-allergic effects, and regulates water-salt metabolism. A large amount of glucocorticoids in vitro can cause imbalance of hormone ratio, glucose metabolism and inorganic salt metabolism in the body; long-term use of glucocorticoids can induce or aggravate infection, cardiovascular system complications, osteoporosis, muscle Atrophy, delayed wound healing and complications of digestive system, such as increased gastric acid, pepsin secretion, inhibition of gastric mucus secretion, reduced resistance of the gastrointestinal mucosa, and even gastrointestinal bleeding or perforation. In addition to many side effects on human health, glucocorticoids also affect the physical state of athletes when they participate in competitions, which violates the principle of fair competition in sports competitions. Therefore, these drugs belong to the International Olympic Committee to announce the ban on synthetic steroid stimulants. In recent years, the detection of glucocorticoids in health foods, nutritional supplements and some animal foods for sports mobilization has occurred frequently. Many athletes have appeared due to eating such contaminated foods. Unnecessary positive results. In addition to strict stimulant testing for participating athletes in the 2008 Beijing Olympic Games, strict monitoring of all types of food for athletes is also required. This article uses Waters HLB solid phase extraction purification technology and UPLC®/TQD system to establish rapid, high-throughput screening and confirmation techniques for glucocorticoids in animal foods, providing a full solution for stimulant monitoring of such foods. Technical support.
experimental method
Reagents and materials
20-well vacuum extraction unit, rotary evaporator, Oasis HLB (500 mg, 6 cc); acetonitrile, methanol, formic acid, and ethyl acetate were chromatographically pure. The experimental water was ultrapure water and anhydrous sodium sulfate. Glucocorticoid standards: prednisone, prednisolone, hydrocortisone, cortisone, methylprednisone, betamethasone, dexamethasone, beclomethasone, fludrocortisone, purity ≥98%.
Liquid chromatography conditions
Liquid Phase System: Waters ACQUITY UPLC
Column: ACQUITY UPLC BEH C18
2.1mm x 50mm, 1.7 μm
Column temperature: 30 ÌŠC
Flow rate: 500 μL/min
Mobile phase A: Water mobile phase B: Acetonitrile gradient: Time 0 min 20% B
Time 5 min 25% B
Time 5.3 min 50% B
Time 5.5 min 60% B
Time 5.6 min 20% B
Time 6.5 min 20% B
Total analysis time 6.5 min Injection volume: 10 μL
Mass spectrometry condition
Mass Spectrometry System: Waters ACQUITY® TQD Detector Ionization Mode: ESI(-)
Capillary voltage: 2.8 kV
Desolvation gas temperature: 400 ÌŠC
Solvent flow rate: 800 L / h
Ion source temperature: 130 ÌŠC
Data Acquisition: Multiple Response Monitoring (MRM)
Collision gas: argon 3.2 x 10-3 mba
Adjusting the ACQUITY TQD allows the parent and daughter ions to have a resolution of 0.7 Da at half width. See Table 1 for a list of multiple reaction monitoring (MRM), dwell time, cone voltage, and collision energy for this experiment. The underlined ion pairs are used for quantitative analysis.
Data acquisition and processing method
Waters MassLynxTM 4.1 operating software for data acquisition and TargetLynxTM application management software for data processing.
Sample preparation
Weigh 5 g of uniformly processed pork samples, add 10 g of anhydrous sodium sulfate, 20 mL of ethyl acetate, mix well and homogenize for 1 min, shake for 10 min, and centrifuge at 10,000 rpm for 5 min. The supernatant was transferred to a concentrate bottle, and 10 mL of ethyl acetate was added to the residue. The above operation was repeated once, and the extracts were combined and evaporated to dryness at 40 ° C. After concentration, the sample was dissolved in 5 mL of 30% aqueous methanol. Oasis HLB was activated in advance with 3 mL of methanol and 6 mL of water. The above extracts were all passed through a column, washed successively with 5 mL of water, 5 mL of 50% aqueous methanol solution, and then drained. Elute and receive with 10 mL of methanol/acetonitrile (1:1). The eluate was dried under nitrogen at 40 °C, and dissolved to 1 mL with acetonitrile/water (2:8). The filter was passed through 0.2 μm. After the machine analysis and determination.
Results and discussion
Determination of sample pretreatment conditions
Glucocorticoids belong to a class of fat-soluble hormones and are structurally all cyclopentane polyhydrophenanthrene derivatives. Therefore, the use of ethyl acetate as an extraction solvent can effectively extract such drugs from animal tissues. Due to the strong non-polar nature of these compounds, there is a strong non-polar retention effect on the HLB column, so the experiment uses a low elution strength methanol/water to dissolve the sample extract and transfer it onto the HLB column, followed by water and 50 % aqueous methanol solution is used to remove polar and medium-polar sample matrix impurities, and then the glucocorticoid drug is eluted with a methanol/acetonitrile mixed solvent, and some non-polar impurities such as animal fats remain on the HLB. Effective purification and enrichment of glucocorticoids.
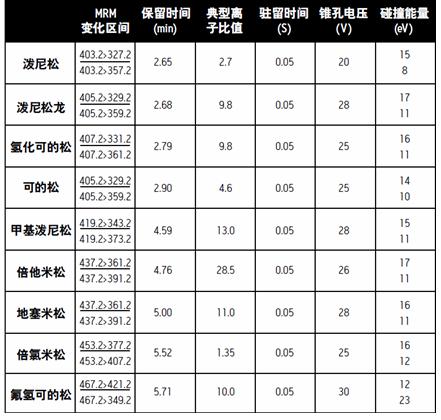
Table 1. ACQUITY TQD MRM parameters.
Optimization of chromatographic conditions
Since there are some differences in the polarity of the nine glucocorticoid compounds, the gradient method was used to separate the acetonitrile/water mobile phase system. The results are shown in Figure 1. Among them, prednisolone and cortisone (405.2 > 329.2, 405.2 > 359.2), betamethasone and dexamethasone (437.2 > 361.2, 437.2 > 391.2), the parent and daughter ions of the two groups of compounds are exactly the same, only in the structure It is spatially heterogeneous and cannot be distinguished by mass spectrometry. Therefore, it is necessary to perform quantitative and qualitative analysis after separation by chromatography. The gradient program, column temperature and flow rate are optimized to achieve baseline separation between the two groups, which enables quasi-deterministic and quantitative determination.
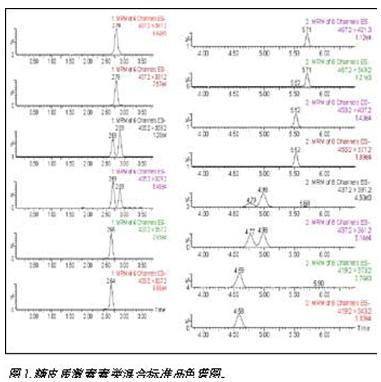
Optimization of mass spectrometry conditions
In ESI(-) mode, the glucocorticoid compound forms a molecular ion peak in the form of [M-H+HCOOH]-, and by collision treatment, the daughter ion scan further obtains quantitative and auxiliary qualitative ion fragment peaks, and then adopts the MRM mode. For monitoring, see Table 1 for MRM conditions. The experiments were carried out to optimize the parameters such as capillary voltage, cone voltage and collision energy, so that the sensitivity and resolution of the method can meet the detection needs.
Linear range, accuracy and precision of the method
Nine glucocorticoid mixed standards were injected in the range of 1 ng/mL ~ 100 ng/mL to obtain linear equations and correlation coefficients. The addition recovery and RSD were calculated by adding blank samples at 10 μg/kg level and paralleling 6 times. The results are shown in Table 2 and Figure 2.
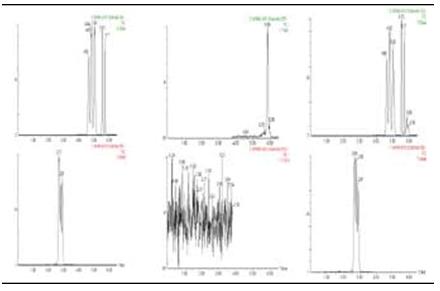
Figure 2. Glucocorticoid standard, blank sample, total sample ion flow (TIC) chromatogram.
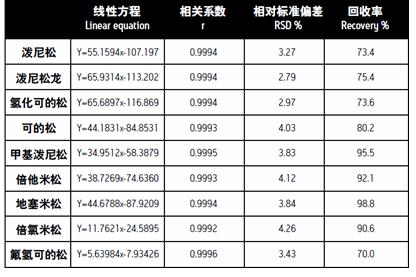
Table 2. Linear equations, correlation coefficients, relative standard deviations, and recoveries for glucocorticoid stimulants.
in conclusion
In this paper, HLB purification and UPLC/TQD system were used to establish a rapid and effective comprehensive analysis program for 9 glucocorticoids in animal foods. Oasis HLB can effectively purify and enrich animal samples, remove matrix interference such as oil and protein, improve method sensitivity and reduce matrix effect. UPLC/TQD system achieves efficient separation and rapid determination within 6.5 min. Achieve accurate quantification and confirmation purposes. It provides powerful technical support and reference for the monitoring of glucocorticoids in Olympic animal foods, and also provides meaningful reference for the monitoring of other types of foods.
references
• Vaerie A. Frerichs, Kathleen M. Tornatore. Journal of Chromatography B, 802 (2004) 329-338.
• Van den hauwe, F. Dumoulin, JP Antignac, MP Bouche, C. Elliott, C. Van Peteghem. Analytica Chimica Acta, 473 (2002) 127-134.
• Olivia Van den hauwe, Manuela Schneider, Ali Sahin, Carlos H. Van Peteghem. J. Agric. Food Chem, 51 (2003) 326-330.
|
You are always welcome to visit our company and taste our products. Contact: MS. Sunny Wang |
|
| Product Description | |
| Name | Canned Saury |
| Flavor | Brine, Oil, Tomato Sauce |
| Type | Bone-in and skin-on, bone-less and skin-less |
| Certificates | EU, FDA, BRC, HALAL,HACCP,KOSHER |
| Net weight | 125g, 155g, 400g, 417g, 425g, 1kg, 1.88kg. |
| Brand | Our brand or OEM, ODM |
| Shelf life | 3/4 Years |
| MOQ | 1X20'FCL |
| Payment terms | T/T, L/C |
| Delivery time | 25 days after label artwork confirmed and advance payment done. |
| Packing | normal lid or easy open,paper label or lithio can, paper carton or shrinked by tray |
| EU NO. | 3302/01034 |
| RUSSIA NO. | 3302/01034 |
| Shipping docs | Commercial Invoice |
| Packing List | |
| Bill of Lading | |
| Certificate Of Origin/ Form A | |
| Health Certificate | |
| Veterinary Certificate | |
| Catching certificate | |
| Or as per customer`s request | |
Canned Fish,Saury In Brine,Canned Saury Fish,Canned Saury In Brine
Tropical Food Manufacturing (Ningbo) Co., Ltd. , https://www.tropical-food.com