Paul D. Rainville and Robert S. Plumb
Waters Corporation, Milford, MA, USA
Application advantage
This new tandem quadrupole mass spectrometer system greatly increases the sensitivity of drug compound analysis for low-concentration tissue samples.
Waters Solutions
XevoTM TQ-S
MassLynxTM in conjunction with TargetLynxTM
ACQUITY UPLC ®
ACQUITY UPLC BEH Column Chemistry
Key words
StepWaveTM, T-Wave, TM Bioanalysis, Pharmaceutical Compounds, Fluticasone Propionate, Salmeterol Succinate
Introduction
Accurate quantification of drugs in biological fluids helps to accurately determine the pharmacokinetic parameters of the drug. Compounds in low-exposure tissue samples (eg, inhaled or strongly metabolized drugs) require very sensitive analyses to accurately determine the elimination phase of the pharmacokinetic profile. This is a challenge for the sensitivity of modern LC/MS/MS instruments.
The Xevo TQ-S is an ultra-high sensitivity tandem quadrupole mass spectrometer. It features StepWave ion transport technology featuring an innovative off-axis ion source design. This design significantly increases the ion transport efficiency from the ion source to the quadrupole analyzer, while its off-axis ion path also removes neutral contaminants. The combination of these two features greatly increases the sensitivity of the LC/MS/MS system.
In this application note, we describe the increased sensitivity of two classes of drug compounds, fluticasone propionate and salmeterol succinate, which are inhaled drugs and exhibit very low levels in the human circulatory system. concentration. The Xevo TQ-S's high data capture rate feature allows rapid collection of large numbers of multiple reaction monitoring (MRM) migration ions while simultaneously acquiring full scan/MRM data.
experiment
Chromatographic conditions
Liquid Chromatography (LC) System: ACQUITY UPLC (Dual Solvent Management System, Sample Manager, HT Column Incubator)
LC column: ACQUITY UPLC BEH C 18 column, 1.7 micron, 2.1 × 50 mm
Gradient: A : 0.1 % ammonia ( Aq ) B : methanol 5 ~ 95% B eluted for more than 1.5 minutes
Flow rate: 600 μl/min
Injection volume: 10 μl
Mass spectrometry condition
Mass Spectrometry System: Xevo TQ-S and Xevo TQ operate in electrospray positive ion mode
MRM data collection: 501 => 292.95 Fluticasone
416.15 => 380 salmeter
Voltage: Capillary, cone and collision voltages are optimized for each mass spectrometer and cone flow
Source temperature: 140 °C
Solvent removal temperature: 625 °C
Spray gas flow: 1200 liters / hour
Data management
MassLynx 4.1
Quantitative analysis using the TargetLynx application management system
Results and discussion
Accurate quantification of pharmaceutical compounds in biological fluids by virtue of the ability to recognize target peaks from background noise. The StepWave source in the Xevo TQ-S is designed to optimize this feature. It includes two differentially-extracted ion transport stages; both stages enable T-Wave 1 technology (multilayer ring RF units). When the ion beam passes through the source sampling hole, the ion beam will diffuse to some extent. Because StepWave's inlet is designed to be large enough, it can effectively capture all the ions in the diffuse ion cloud. The first stage of the design ensures that all ions are effectively aggregated and enter the second stage. This off-axis design ensures that neutrals entering the source sampling orifice are actively removed from the system (as shown in Figure 2).

In Figure-2, we can see how the ion cloud diffuses as it enters the MS migration zone and then aggregates into a narrow ion beam as it moves to the quadrupole analyzer zone. Neutral compounds that are not charged are discharged and discarded because they cannot pass through this off-axis path. This greatly increases the detection limit, allowing bioanalysts to quantify the analytical components of plasma, serum and urine at levels that were previously unattainable.
With this StepWave design, we found that the sensitivity increased by about 10 to 25 times based on the compound being analyzed. For example, modern drugs that treat asthma and rhinitis (such as beta 2 agonists and steroid hormones) do not enter the circulatory system and are usually administered by inhalation. These drug compounds are intentionally present at very low concentrations in the body's systemic system and are therefore suitable for analysis using the Xevo TQ-S system.
Fluticasone propionate and salmeterol succinate were dissolved in methanol and injected into the plasma at different physiologically relevant levels. Thereafter, the plasma sample was precipitated with 2:1 acetonitrile and centrifuged, and the supernatant after centrifugation was injected into the chromatographic system.
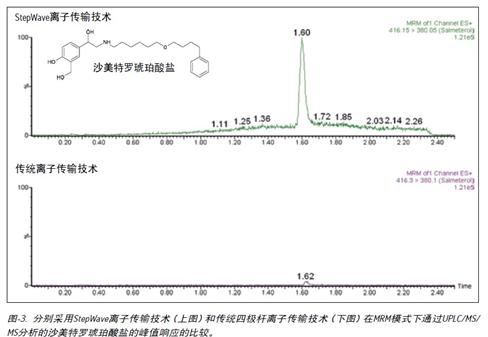
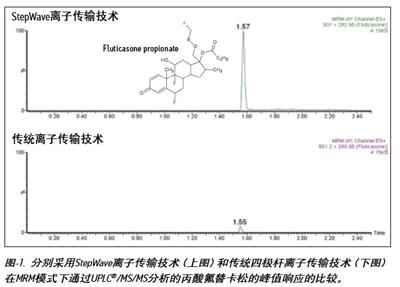
The data shown in Figure-1 shows an increase in sensitivity when analyzing fluticasone propionate using Xevo TQ-S; the data in Figure 3 shows an increase in peak response to salmeterol succinate analysis. The peak height of fluticasone propionate increased by a factor of 12 and the peak response of salmeterol succinate increased by a factor of 15.
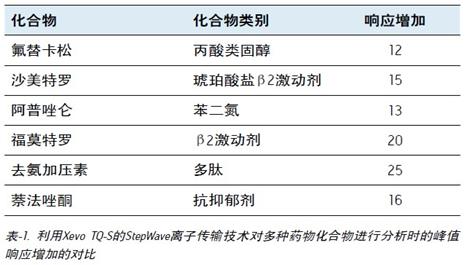
The overall increase in peak response of these two classes of drug compounds and other types of drugs is given in Table-1. Here we can see that the peak response has increased by 12 to 25 times.
In addition to increasing peak sensitivity, there are other advantages. The larger peak response allows the detection limits to be acquired without the need for complex method development, resulting in faster method development. The increase in peak height also simplifies peak integration, making analysis reproducibility better in general applications.
in conclusion
The StepWave ion transport technology in the Xevo TQ-S significantly increases the efficiency of ion sampling in the ion source. Its innovative off-axis geometry also prevents neutral compounds that you don't need from entering the analyzer stage of the instrument. This new design results in a significant increase in sensitivity, ranging from 10 to 25 times. The increase in sensitivity of the analysis has the following functions:
* Increase the detection limit of low exposure compounds
* Accelerated method development
* Simplify peak integration
* Better analytical reproducibility
1 Traveling Wave devices described herein and U.S. Patent No. 5,206,506; 1993 similarly as described in Kirchner.
Waters, ACQUITY UPLC, and UPLC are registered trademarks of Waters Corporation. Xevo, StepWave, T-Wave, MassLynx, TargetLynx, and The Science of What's Possible are trademarks of Waters Corporation. All other trademarks are the property of their respective owners.
Hydrolyzed Sponge White Powder
Sponge
Chengdu Sino Tech company , https://www.cnherbfun.cn